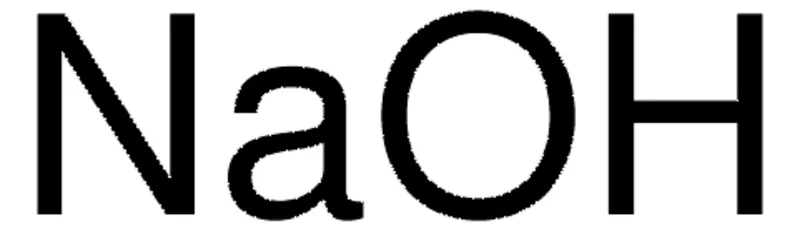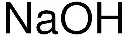General description
Sodium hydroxide (caustic soda) is an inorganic source of
hydroxide ions that can be used to alkylate phenols, alcohols, aldehydes, and
ketones. NaOH can also be used to convert amides into amines, to produce
dichlorocarbenes, and in saponification of carboxylic acid derivatives.
Application
Sodium hydroxide may be used:
- As a
deprotonator in the synthesis of nanoparticles of zeolitic imidazolate
framework (ZIF-94).
- As a
precipitating agent in the synthesis of NiO (nickel oxide) nanoparticles
using nickel nitrate.
- As a
pH controller in the synthesis of zinc chromite (ZnCr2O4)
oxide powder from zinc nitrate and chromium nitrate by hydrothermal
process.
Analysis Note
Assay (acidimetric,NaOH): ≥ 99.0 %
Carbonate (as Na₂CO₃): ≤ 1.0 %
Chloride (Cl): ≤ 0.012 %
Phosphate (PO₄): ≤ 0.0005 %
Silicate (SiO₂): ≤ 0.001 %
Sulfate (SO₄): ≤ 0.010 %
Total nitrogen (N): ≤ 0.0003 %
Heavy metals (as Pb): ≤ 0.0005 %
Al (Aluminium): ≤ 0.0005 %
As (Arsenic): ≤ 0.0001 %
Ca (Calcium): ≤ 0.0005 %
Cu (Copper): ≤ 0.0002 %
Fe (Iron): ≤ 0.0005 %
K (Potassium): ≤ 0.05
Mg (Magnesium): ≤ 0.0005 %
Ni (Nickel): ≤ 0.00025 %
Pb (Lead): ≤ 0.0005 %
Zn (Zinc): ≤ 0.001 %
Legal Information
EMSURE is a registered trademark of Merck KGaA, Darmstadt,
Germany